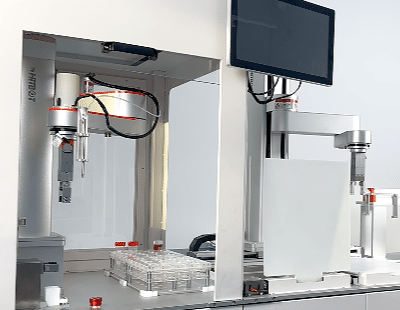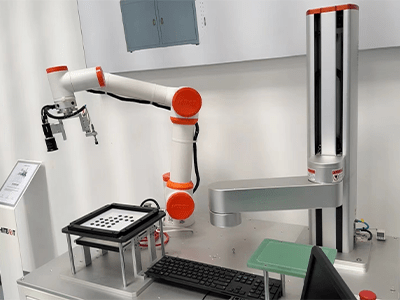
Collaborative robots for medical device manufacturing
Precision Partners: How Collaborative Robots Are Transforming Medical Device Manufacturing
The medical device industry faces a unique challenge: producing life-saving technologies with microscopic precision under intense regulatory scrutiny, often in small, complex batches. Traditional automation solutions were often too rigid, expensive, or unsafe for close human interaction. Enter the collaborative robot (cobot) – a game-changer bringing unprecedented flexibility, precision, and efficiency to this critical sector. Let’s explore why cobots are becoming indispensable partners on the cleanroom floor.
What’s the Medical Device?
A medical device is any instrument, apparatus, machine, or implant that is designed for medical purposes. These devices are used to diagnose, prevent, monitor, or treat medical conditions. They can range from simple tools like thermometers and bandages to complex machinery like MRI scanners, pacemakers, and surgical robots. Medical devices are essential in healthcare, as they aid in improving patient outcomes, enhancing diagnosis accuracy, and supporting treatments. Their design and production are strictly regulated to ensure safety and effectiveness for patient care.
Why Medical Device Manufacturing Needs Cobots
Medical device makers operate under extraordinary pressures:
- Razor-Thin Tolerances: Components for implants, surgical tools, or diagnostics often require sub-millimeter accuracy.
- Stringent Sterility & Cleanliness: ISO Class 5-8 cleanrooms demand contamination-free processes.
- Complex, Low-Volume/High-Mix Production: Frequent product changes and small batch sizes are common.
- Relentless Regulatory Oversight (FDA, ISO 13485): Every process step must be meticulously documented and validated.
- Skilled Labor Shortages: Finding and retaining workers for highly repetitive, delicate tasks is difficult.
- Cost Pressure: Innovation is expensive; manufacturing efficiency is paramount.
Traditional industrial robots struggled here. They required safety cages (taking up precious cleanroom space), complex programming for frequent changeovers, and massive upfront investment – often only justified for huge volumes. Cobots bridge this gap perfectly.
Cobots: The Ideal Surgical Assistants for Production
Unlike their bulky, caged predecessors, cobots are designed from the ground up for safe, side-by-side work with humans. Key features making them ideal for medical devices:
- Inherent Safety: Force-limited joints, rounded edges, and advanced sensors allow them to stop immediately upon unexpected contact, eliminating the need for bulky safety cages in many applications (after proper risk assessment).
- Unmatched Flexibility & Ease of Programming: Intuitive hand-guiding or simple graphical interfaces allow technicians (not just robotics engineers) to quickly reprogram cobots for new tasks or product variants – essential for high-mix, low-volume production.
- Space Efficiency: Compact designs fit easily into existing cleanroom work cells or benchtop setups without major facility redesign.
- Precision & Repeatability: Modern cobots offer accuracy down to +/- 0.03mm or better, crucial for assembling micro-components or performing delicate tasks.
- Cleanroom Compatibility: Many cobot models are available with ISO Class 5 certified cleanroom variants featuring special lubricants, sealed joints, and smooth, easy-to-clean surfaces that minimize particle generation.
- Lower Total Cost of Ownership (TCO): Significantly lower upfront costs, faster deployment, easier programming, and reduced integration complexity make cobots financially viable even for smaller batches and SMEs.
- Seamless Data Logging: Integrated systems facilitate detailed process data collection, essential for regulatory documentation (e.g., FDA 21 CFR Part 11 compliance for electronic records).
Key Applications Revolutionizing Medical Device Production
Cobots are enhancing quality and efficiency across the manufacturing lifecycle:
- Micro-Assembly & Precision Handling:
- Inserting minute components (e.g., sensors in catheters, springs in surgical staplers, lenses in endoscopes) with sub-millimeter accuracy.
- Handling delicate, sterile, or expensive components (silicon wafers, brittle plastics, active pharmaceutical ingredients – APIs) without damage or contamination.
- Applying microscopic amounts of adhesives or sealants consistently for hermetic sealing of implants or devices.
- Inspection, Testing & Quality Control (QC):
- Automated visual inspection (AVI): Using integrated vision systems (2D/3D) to check for defects (cracks, debris, misalignments), verify component presence, or read tiny serial numbers/barcodes with superhuman consistency.
- Functional testing: Performing repetitive test sequences (button presses, switch actuations, flow rate tests) precisely and recording results automatically.
- Leak testing: Precisely maneuvering devices into test chambers and initiating test sequences.
- Lab Automation & Process Steps:
- Liquid handling: Precise pipetting, sample preparation, or reagent dispensing in diagnostic kit manufacturing or R&D labs.
- Weighing & dispensing: Handling precise amounts of powders or liquids for drug-eluting devices or combination products.
- Machine Tending: Loading/unloading CNC machines, injection molders, or laser marking systems used for device components, ensuring consistent cycle times.
- Packaging & Kitting:
- Primary packaging: Placing delicate devices into blister packs, clamshells, or vials with gentle precision.
- Secondary packaging & palletizing: Assembling kits (device + accessories + instructions) and preparing finished goods for shipment.
- Surface Treatment & Finishing:
- Polishing & Deburring: Consistently finishing complex surfaces on implants or surgical tools with controlled force.
- Cleaning & Sterilization Support: Handling components through various cleaning or sterilization baths.
Addressing the Regulatory Hurdle: Compliance is Key
Regulatory compliance isn’t optional; it’s existential. Cobots, when implemented correctly, can be powerful compliance enablers:
- Process Consistency & Repeatability: Eliminates human variability, a major source of non-conformance. Every movement is identical.
- Comprehensive Data Traceability: Cobot controllers and integrated sensors generate detailed, time-stamped logs of every action, force applied, vision result, etc. This data is invaluable for process validation (IQ/OQ/PQ) and audit trails.
- Reduced Contamination Risk: Cleanroom cobots minimize human handling and particle generation. Automated processes within closed systems further enhance sterility assurance.
- Validation Support: Reputable cobot manufacturers provide documentation packages (e.g., Safety Certificates, RoHS/REACH compliance) to support validation efforts. Software features like user access controls aid in electronic signature compliance (21 CFR Part 11).
- Change Control: Reprogramming a cobot for a validated process change is often faster and more rigorously documented than retraining multiple human operators.
Implementing Cobots Successfully: A Practical Guide
Moving from concept to cleanroom integration requires planning:
- Identify High-ROI Opportunities: Focus on tasks that are:
- Highly repetitive and prone to human error
- Requiring superhuman precision or consistency
- Ergonomic nightmares causing worker strain
- Bottlenecks in production flow
- Critical for quality/conformance data capture
- Select the Right Cobot & Tooling:
- Payload & Reach: Match to component weight and workspace size.
- Accuracy & Repeatability: Ensure specs meet the task’s demands.
- Cleanroom Rating: Mandatory for sterile areas (ISO Class 5-8).
- End-of-Arm Tooling (EOAT): This is critical! Choose grippers (vacuum, electric, soft robotic), force sensors, or vision cameras specifically designed for medical components and clean environments.
- Safety Assessment: Conduct a thorough risk assessment (ISO 10218, ISO/TS 15066) for each application. Collaborative doesn’t automatically mean safe in all contexts; additional safety measures (speed/force limits, area scanners) might be needed.
- Focus on Seamless Integration:
- Work Cell Design: Optimize layout for human-cobot interaction and material flow.
- Software & Connectivity: Ensure compatibility with MES/SCADA systems for data exchange. Utilize intuitive programming interfaces.
- Validation Protocol: Develop a comprehensive plan covering Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) specifically for the cobot system in its intended use. Document everything meticulously.
- Upskill Your Workforce:
- Train technicians on programming, operation, and basic maintenance.
- Emphasize the cobot as a tool to augment their skills and remove drudgery, not replace them. Foster collaboration.
The Future: Smarter, More Connected Cobots
The evolution continues rapidly:
- Enhanced AI & Machine Vision: Cobots will better handle unpredictable variations in parts or environments, perform more complex inspections autonomously, and learn optimal paths.
- Advanced Force Sensing: More sophisticated control for ultra-delicate assembly and feedback during processes like polishing.
- Tighter MES/ERP Integration: Real-time production data flowing seamlessly for better analytics and traceability.
- Mobile Cobots (Cobots on AMRs): Autonomous mobile manipulators bringing automation to different points on the production line.
- Simpler Programming & RaaS (Robotics as a Service): Lowering barriers further with no-code/low-code options and subscription models.
- Specialized Medical EOAT: Continued innovation in grippers designed specifically for handling sterile, delicate, or uniquely shaped medical components.
Conclusion: Partnering for Precision and Patient Safety
Collaborative robots are no longer a futuristic concept in medical device manufacturing; they are a present-day necessity for companies striving for excellence. By tackling the industry’s core challenges – precision, flexibility, compliance, cleanliness, and cost-effectiveness – cobots empower manufacturers to:
- Elevate Quality & Consistency: Produce medical devices meeting the strictest tolerances, batch after batch.
- Accelerate Time-to-Market: Respond faster to design changes and scale production efficiently.
- Strengthen Regulatory Compliance: Generate irrefutable process data and ensure consistent execution.
- Protect Valuable Personnel: Free skilled workers from repetitive, ergonomically challenging tasks for higher-value work.
- Enhance Competitiveness: Achieve sustainable manufacturing efficiency and agility.
In an industry where precision directly translates to patient safety and outcomes, the partnership between human expertise and collaborative robotic precision is proving to be transformative. Embracing cobots isn’t just about automation; it’s about building a future of more reliable, accessible, and life-changing medical technologies.
Is your medical device manufacturing ready for a collaborative advantage? Contact Our Automation Specialists for a free consultation on identifying the right cobot applications for your precision processes. Explore our Medical-Grade Cobot Solutions designed for cleanrooms and rigorous compliance demands. Download our whitepaper: “Validating Cobot Systems for FDA-Compliant Medical Manufacturing”.